International and Kazakhstan experience of the single distribution of medicines
GLOBAL AND KAZAKHSTAN EXPERIENCE OF MEDICINAL PROVISION
We present to your attention a publication from a series of analytical reviews of the Single Distributor "SK-Pharmacy" LLP within the framework of the National Project "Health of the Nation".
The pandemic has shown how acute and urgent the issue of medicinal provision can become in emergency situations, when certain drugs are suddenly required in large volumes.
Healthcare is the second largest area of expenditure for governments (more than 9% of GDP). The level of medicinal safety of the country depends on how effectively the system of public procurement of medicines is built, the key factor of which is the continuity and sufficiency of drugs for all citizens, including in conditions of exponential demand. Various mechanisms are used to reduce procurement prices in the world: long-term contracts, direct negotiations with manufacturers, risk-sharing, centralized/joint procurement – they can all be considered promising tools for modernizing the current system of public procurement and medicinal supply.
One of the significant tools for preventing shortages may be the transition to a centralized procurement system, which should facilitate the possibility of planning and interregional distribution of medicines according to needs, strengthen the state's negotiating position on price reduction issues and increase patient coverage by the state.
Global procurement initiatives
Global procurement practice shows that centralized procurement as an important social function in the world is carried out, as a rule, by organizations that are part of or associated with authorized bodies in the field of healthcare. In Argentina, India, New Zealand, Estonia, centralized procurement at the state level is carried out by the Ministry of Healthcare. In reference models that are similar to the RK in a number of important criteria, centralized procurement is carried out by state structures or procurement agencies subordinate to state structures.
In Australia, the centralized procurement of medicines is carried out by a Procurement Agency subordinate to the Australian Healthcare Administration; in Denmark - by a Procurement Agency subordinate to regional authorities, like in Norway; in Serbia - by the National Compulsory Health Insurance Fund subordinate to the state; in Saudi Arabia - by a Procurement Agency financing from the state budget and by the profit generated by the agency due to the mark-up on medicines; in USA - by pharmaceutical distributors who are the leaders of the global wholesale market - McKesson, AmerisourceBergen and Cardinal Health consolidated more than 90% of sales of the American market and about 45% of sales of all pharmaceutical products in the world.
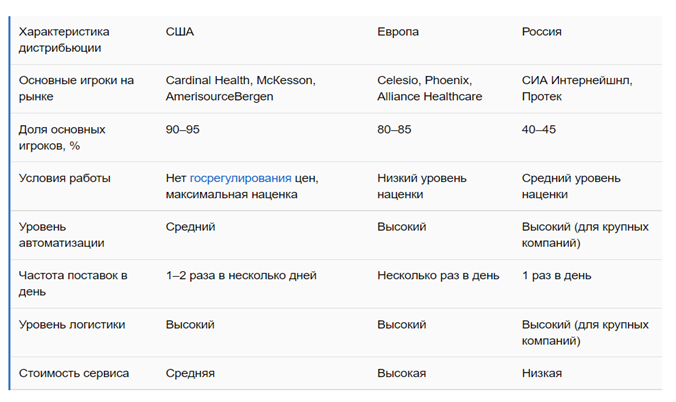
Pharmaceutical distribution in the USA, Europe and Russia
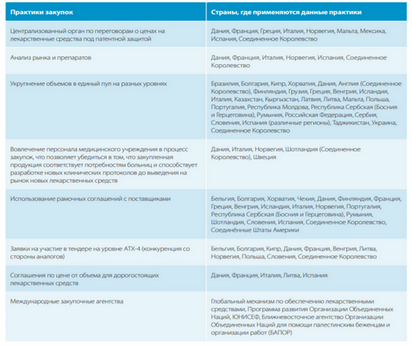
Procurement practices that can guarantee more competitive prices by improving the provision of medicines to patients
The generally accepted bases for realization of procurement and order distribution are formulated in a number of international documents, such as, for example, EU Directives, the Multilateral Agreement on Public Procurement within the WTO, documents of the Organization of Asia-Pacific Economic Cooperation. International organizations are developing mechanisms for realization of centralized procurement of goods and services for the needs of their units, as well as for assistance to participating countries in their fight against the COVID-19 pandemic.
In order to conduct procurement of goods and services, the United Nations formed a special platform in 1990 – the UN Global Market (UNGM). The UN market consists of many specialized agencies, affiliated programs, funds and various subsidiary bodies.
In 1999, the Interdepartmental Pharmaceutical Coordination Group (IPC), with the participation of UNICEF, the United Nations Population Fund (UNFPA), WHO and the World Bank, developed and implemented four strategic goals and twelve principles of activity in the field of good practices of procurement medicines. One of them is to achieve as low as possible total costs. The Governments of the 24 EU member states have launched an interaction plan to conduct joint procurement of pandemic vaccines. The project "Healthy Aging - Public Procurement of Innovations (HAPPI)", aimed at combining the efforts of European public authorities engaged in procurement in the field of healthcare for their joint activities, was also supported.
In April-July 2015, Belgium, the Netherlands and Luxembourg signed a Memorandum of Understanding for jointly discussion of prices for drugs for the treatment of orphan diseases, and Bulgaria and Romania announced their intention to make jointly procurement of high-cost medicines. The joint Initiative of the Benelux countries and Austria (BeNeLuxA Initiative, to which Ireland joined in 2018) on medicine policy, launched in 2015, has a number of achievements in its asset, including the coordination of the price of nusinersen with "Biogen" company in 2018.
Also in 2015, the Nordic Pharmaceutical Forum (Nordisk Lægemiddel Forum), bringing together Denmark, Iceland, Norway and Sweden, started working.
Cooperation between Bulgaria and Romania on the procurement of high — cost medicines also begins in 2015, and in June 2016, 9 more countries - Croatia, Estonia, Hungary, Latvia, Macedonia, Poland, Serbia, Slovakia and Slovenia by signing the Sofia Declaration joined them.
In 2015, the Scandinavian Pharmaceutical Forum for the purpose of cooperation in the field of procurement of medicines was also established.
In July 2016, the Council of the European Union issued a number of recommendations that promote the affordability of medicines and medicinal provision. There is a recommendation on voluntary cooperation between the relevant public authorities of each of its member countries among them.
In September 2016, the WHO European Office indicated that the procurement practice, which can guarantee more competitive prices and improve the provision of medicines to patients, implies:
- consolidation of volumes in a single pool at different levels (Brazil, Bulgaria, Cyprus, Croatia, Denmark, Finland, Georgia, Greece, Hungary, Iceland, Italy, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Malta, Republic of Moldova, Poland, Portugal, Romania, Scotland, Serbia, Slovenia, Republika Srpska, Tajikistan, Ukraine, United Kingdom);
- the use of framework agreements (Belgium, Bulgaria, Croatia, Denmark, Finland, France, Greece, Hungary, Iceland, Italy, Norway, Portugal, Romania, Scotland, Slovenia, Republika Srpska, United Kingdom, USA)
- conducting procurement through international procurement agencies (the global mechanism for the provision of medicines, UNDP, UNICEF, UNRWA).
In May 2017, Cyprus, Greece, Italy, Malta, Portugal and Spain signed the Valletta Declaration on the evaluation and procurement of new medicines. Croatia, Ireland, Romania and Slovenia joined them later.
In June 2018, representatives of the national healthcare technology assessment agencies of Finland, Norway and Sweden signed a memorandum on the establishment of FINOSE cooperation for joint assessment of clinical and economic efficiency.
In 2019, China launched a realization of pilot program of centralized procurement and use of medicines to significantly reduce the cost of medicines for the population. To date, the fifth round of group procurement of medicines has already been held. Medicines selected by the Chinese government within the framework of the fifth round of the new centralized medicine procurement program will be on average 56% cheaper than usual for those state medical institutions that purchase them, according to the National Healthcare Security Administration of the PRC.
During the COVID-19 pandemic, the United Nations Children's Fund (UNICEF) annouced a tender for the supply of personal protective equipment (PPE) to meet the needs of organizations such as WHO, IAEA, UNDP, UNICEF, the International Organization for migration, the International Federation of Red Cross and Red Crescent societies. An important condition of the tender is the ability of suppliers to provide immediately products from warehouses for their operational use in order to resist COVID-19.
The COVID-19 pandemic is putting constant pressure on the production capacities of global manufacturers and global supply chains. Organizations of the UN system have faced the same problems in the field of procurement as individual states: border closures, termination of air traffic, quarantine measures, overpricing, supply that does not meet the level of demand. The main suppliers of PPE for UN organizations were Chinese manufacturers, who, due to the imposed restrictive measures, could not fulfill the terms of contracts. Short-and long-term market problems still persist due to the limited availability of raw materials, as well as uncertainty in the scale of needs and the duration of demand for products.
Back in 2009, the outbreak of H1N1 infection highlighted a number of problems related to the purchasing power of European Union countries and access to vaccines and medicines during pandemics.
In 2010, the European Council instructed the European Commission to start developing mechanism for joint procurement of vaccines in the event of a possible pandemic.
In 2014, the member States of the European Union concluded an Agreement on joint public procurement in the field of medical countermeasures (hereinafter referred to as the Agreement), which became the basis of a mechanism for countering cross – border challenges in the field of healthcare. The Agreement was signed by 37 countries, including all EU and European Economic Area countries, Albania, Bosnia and Herzegovina, Northern Macedonia, Serbia, Montenegro. The United Kingdom remains a party to the Agreement. The purpose of implementing the joint procurement mechanism is to ensure fair access to specific medical countermeasures and increase the security of supplies, as well as to establish more balanced prices for EU member states. Together, states can act as a major customer, which ensures the best prices and allows for priority deliveries to the most needy countries. In order to be prepared for the emergence of a serious cross-border threat in the healthcare sector, EU institutions and countries that have joined the Agreement can participate in the procedure of joint procurement of vaccines, antiviral drugs, diagnostic kits, medical equipment, personal protective equipment, and medical services. The mechanism of joint public procurement is designed to ensure:
• better preparedness of states for pandemics;
• increasing the purchasing power of individual states;
• facilitating the exchange of best practices and expert opinion between countries.
As of April 2020, the European Union has made four purchases related to the COVID-19 pandemic using the joint procurement mechanism: the purchase of medical masks, personal protective equipment, ventilators and diagnostic kits. The total cost of the purchased PPE is 1.5 billion euros, the cost of ventilators is 790 million euros. In total, 25 European countries took part in the procurement. Being at the stage of leaving the EU until December 31, 2020, the United Kingdom also had the right to participate in the procurement, but the government refused the proposed scheme.
In addition, over the past 15 years, supranational / global financing and procurement initiatives related to specific interventions and diseases have had a huge impact on the provision of new high-quality medicines. Global Alliance for vaccines and immunization (Gavi, Vaccine Alliance), Global Drug Facility (GDF), Global Fund to Fight AIDS, Tuberculosis and Malaria, etc. (49-52) belong them. These organizations conduct global tenders for individual drugs, thanks to which they managed to achieve market development and increase the availability of generic drugs characterized by price availability. One of the achievements of the Global Mechanism for providing medicines is to reduce the cost of a 24-month course of treatment for one patient with multidrug-resistant tuberculosis by 26%.
The head of the central procurement agency of the Federal State of Schleswig-Holstein in Germany, among the main lessons of the pandemic in procurement issues, called the need for centralization in the condition of the crisis, as well as the availability of opportunities (technical and legal) for electronic bidding, international cooperation and cooperation between different levels of government (federation and lands). Special difficulties were caused by the issues of coordination of measures, the complexity of the structure of markets (unavailability of goods, materials, disruption of supply chains).
Kazakhstan's medicine provision system
As for Kazakhstan, the response of our republic was the creation of the Single distributor in 2009. The results of ten years of its activity indicate that the decision was more than correct, because significant budget savings were achieved. The availability of medicinal care is an important component of the availability of medical care. In the context of a pandemic and the current global financial and economic crisis, this tool is of particular importance for the economic and social effectiveness of the state in providing medicines.
"SK-Pharmacy", as the Single distributor, provides medicines to medical organizations and the population of the republic within the framework of the GVFMC, the CSHI system, in addition, ensures the development of the pharmaceutical industry by consolidating public procurement of medicines. In total, over the years of operation of "SK-Pharmacy", more than 162.9 billion tenge was saved. This result was favored by the centralization of procurement and the consolidation of the demand volume from all regions.
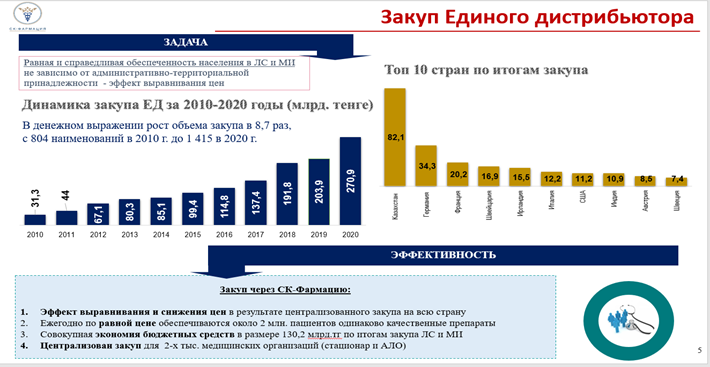
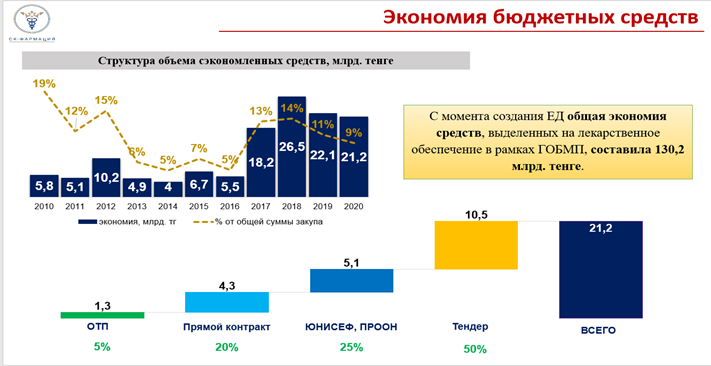
The savings are achieved by centralizing the procurement and consolidating the volume of demand from all regions. Suppliers go to a significant price reduction when the volume is purchased centrally for the whole republic. And this difference between the allocated budget and the amounts actually used is the savings. The released difference of medical organizations is directed to the additional procurement of medicines.
In addition, the Single distributor has effective mechanisms to reduce the price. For example, tax-free drugs are purchased without intermediaries directly from the manufacturers themselves, and also through international organizations. The intermediary is excluded from the purchasing chain. At the same time, part of the logistics costs (certification, customs clearance) is borne by the Single distributor, whereas in normal cases, when goods are purchased through distributors, all such costs are already included in the cost price for procurement through the single distribution system. The practice of concluding direct contracts and international organizations are paying off. This year, the savings only on contracts within the framework of procurement directly from the manufacturer amounted to more than 5.3 billion tenge.
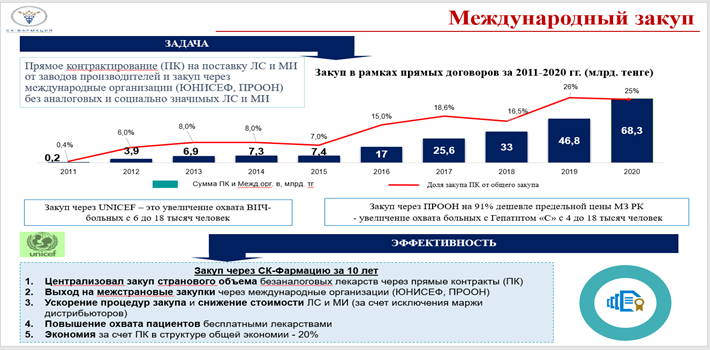
At the same time, the purchase through UNICEF allowed to increase the coverage of HIV patients from 6 thousand to 18 thousand people by reducing the price of specific drugs. Procurement through UNDP is an increase in the coverage of patients with Hepatitis "C" from 4 thousand to 18 thousand people.
Savings based on the results of the procurement are also noted at the procurement under long-term contracts with domestic producers, which suggests that the development of the domestic pharmaceutical industry is not only ensuring the national medicinal safety of the country, but also the possibility of saving budget funds.
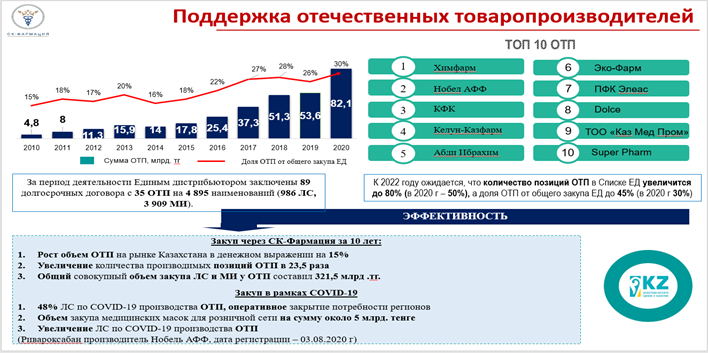
But the greatest price reduction is achieved through an auction to lower the price, which is held as part of two-stage tenders for the purchase of medicines. It is this stage, based on the results of a serious competition between suppliers, that provides a big part of budget savings through procurement procedures.
This is only an actual saving, without taking into account the price jumps that could occur due to the exchange rate difference, when "SK-Pharmacy" acts as a kind of buffer, protecting the population from a shortage in medicines due to price increases, since early tender procedures allow you to keep the same procurement prices for medicines, as well as, in the case of direct contracts with foreign manufacturers, international organizations taking on all currency risks.
Thus, for 10 years of operation, the single distribution system has fully justified itself as a tool for minimizing risks through centralized procurement, restraining the growth of prices for pharmaceutical products within the framework of the GVFMC, reducing the share of intermediaries in the form of local commercial distributors.
In this regard, Kazakhstan, having such a unique state instrument as the Single Distribution, is undoubtedly in an advantageous position. At the same time, WHO fully supports the consolidation of purchases of medicines and medical devices not only within the country, but also works towards strengthening cooperation between countries in conducting joint purchases.
The experience of previous years shows that the single distribution system, through the organization of centralized procurement, minimizes risks in any economic conditions and ensures stability.
The crisis caused by the COVID-19 pandemic has brought significant changes to many areas of life. The sphere of public procurement has not become an exception: the operating conditions have changed, new challenges have formed and traditional challenges have become more acute. Countries are forced to adapt to the current conditions and look for ways to solve (often non-standard) emerging difficulties. In a sense, the current crisis has placed a special responsibility on the public procurement system, because people's lives directly depend on the effectiveness of its functioning in emergency situations.
At the same time, the COVID-19 pandemic may become an additional incentive in the process of reforming the public procurement system. New challenges should be considered as an opportunity for real and fundamental changes, and the reform of public procurement itself should be considered as a catalyst for improving public policy and involving civil society and other stakeholders.
Source: www.sk-pharmacy.kz
- Created on .



